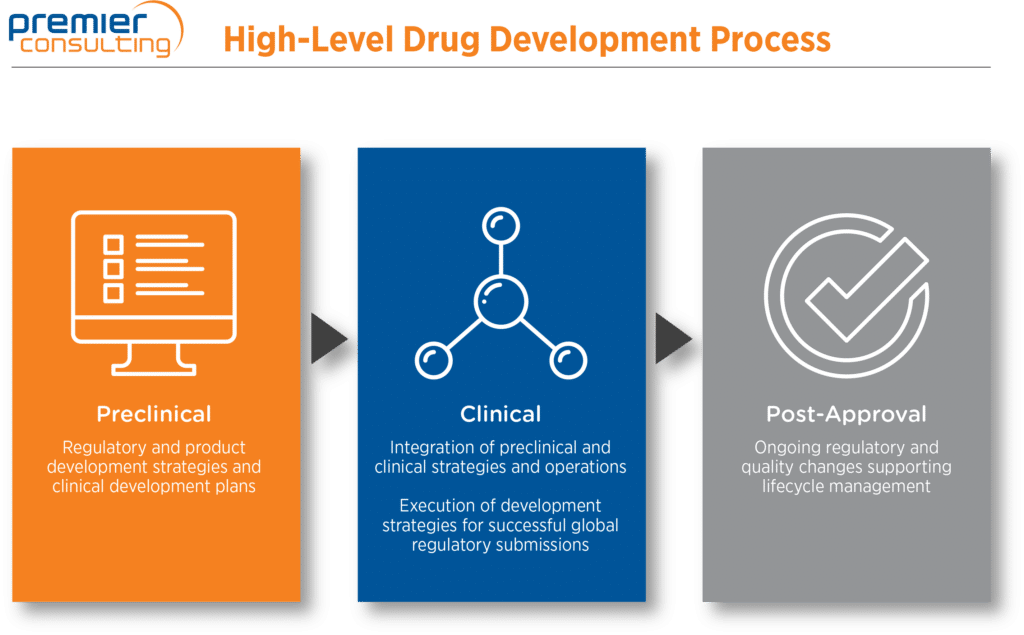
Biotech, MedTech, and small and specialty pharma companies depend on constant vigilance, careful attention to detail, and optimal use of limited resources to realize their products’ life-changing potential. Premier Consulting can help you ascend the challenging path from early discovery through IND application and post-approval life-cycle management.
Whether defining your strategy to achieve FDA buy-in, evaluating the overall appeal of your product concept, prioritizing product opportunities, or assessing a potential investment in an existing asset, we can help guide you throughout the process. Our product development experts, including therapeutic specialists, biostatisticians, and clinical operations professionals, can design a strategy that reduces risk and maximizes the likelihood of commercial success.
Services include:
Product concept evaluation: Starting with a target product profile, we assess the development risks and opportunities to clearly identify your idea’s potential.
U.S. market evaluation: Our experts evaluate your product’s potential by examining current and future competition, unmet needs, marketing requirements, market access considerations, and revenue opportunity, as well as conducting a SWOT analysis.
Business-case insights: We help develop the rationale for asset development or acquisition, including the proposed development outline, potential risks and roadblocks, and valuation.
Product development plan and pre-IND: Look to us for guidance when creating a detailed clinical and development plan, including time- and cost-to-market analysis, FDA feedback, necessary trials, and next steps.
Portfolio analysis: Premier Consulting experts provide a high-level product assessment, product concept analysis, and FDA-vetted development plan to clearly define your path.