Navigating the Global NHP Shortage: How to Keep Your Nonclinical Development Program on Track
In the first half of 2020, as a direct consequence of the ongoing pandemic, China issued a ban on the export of non-human primates (NHPs), including those used for pharmaceutical research. As this affected COVID-19 research, the NIH later issued a Notice of Limited Availability to prioritize studies requiring NHPs and minimize the impact of the resulting shortage.
The Chinese exports ban exposed a challenge for an important segment of drug developers: Large pharmaceutical companies have the power to reserve the limited number of monkeys available in advance, leaving emerging biopharma companies all over the world with a very real and pressing supply chain issue.
An important species for biologics research
Safety studies in animals are an integral piece of the development and eventual approval of new drugs, as they are required by all regulatory agencies around the world. Selecting the correct species is a crucial decision that sponsors must make at the latest when IND-enabling toxicology studies begin.
Monkeys are commonly used due to their genetic and physiological similarities to humans. They are particularly prevalent in studies involving biologics, which are best researched in organisms whose immune systems are similar to ours. Macaques are the most typically chosen species; specifically, rhesus macaques are used primarily in early research, and cynomolgus macaques in later drug development. About 75,000 NHPs are imported to the United States each year. By comparison, Canada imports about 5,000 NHPs each year, mostly cynomolgus macaques used for pharmaceutical research by CROs such as those used by Premier Partners to conduct toxicology studies.
Most NHPs are bred in China
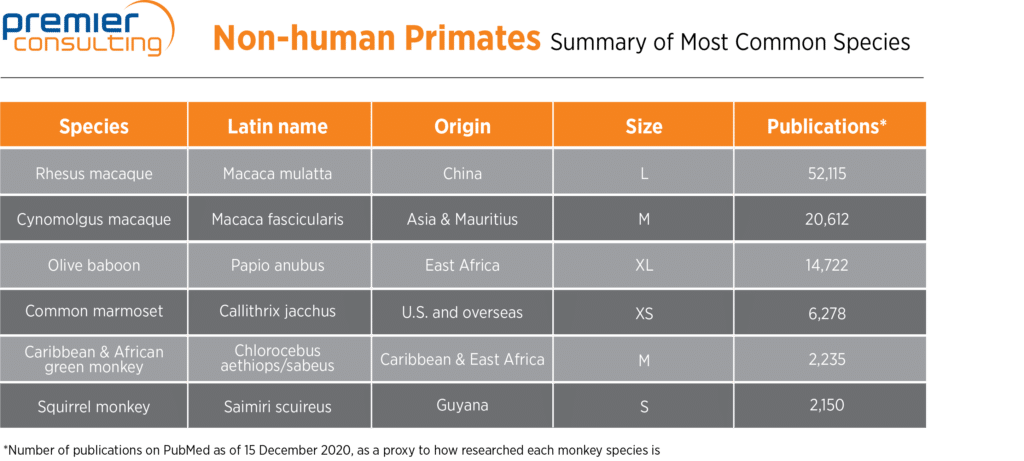
Between 80 percent and 90 percent of the NHPs available worldwide are bred in Asia, including 60 to 80 percent bred in China, since China offers (sub)tropical climates and can provide NHPs with the most suitable growth environment until the animals are ready to be shipped to scientific institutions. This has led to the accumulation of abundant relevant scientific data regarding Chinese-origin NHPs, so sponsors generally prefer them. The table below summarizes the species available from one of the world’s leading providers of monkeys along with their origin, size, and prevalence in research publications.
Sponsors can consider alternative animal models for their nonclinical research. Although each nonclinical (or preclinical) program has its own specific needs, Premier Consulting can suggest several alternatives to Chinese-origin NHPs to those affected by the shortage and to minimize future or sustained supply chain disruptions.
Other sources of cynomolgus macaques
So far, only China has issued a ban on the export of NHPs, so cynomolgus macaques coming from other Asian countries and from the Republic of Mauritius are still available (though demand considerably exceeds supply). U.S. government research comparing physiological and pharmacological parameters and the genetic composition of cynomolgus monkeys from various origins shows, for example, that Mauritius NHPs tend to mature faster than Chinese monkeys and to be more homogeneous, likely an effect of the geographical isolation of the island. This may well be the best midterm solution for a sponsor that has yet to begin safety testing.
Marmosets
Marmosets are an up-and-coming NHP species alternative now being considered by biomedical researchers. They are more primitive than macaques and may, therefore, be the more ethical choice. Their small size — they weigh about as much as a rat — could lead to substantial cost savings. However, more animals may be needed per study to obtain the same toxicokinetic information.
Given that data on marmosets continues to accumulate, the species may prove useful in diversifying the nonclinical research industry supply chain in the long term.
Alternatives to NHPs
Some companies may need to revise their species selection processes in response to the temporary NHP shortage. Many small molecules are still tested in NHPs when alternative non-rodent species could be valid options.
- Minipigs are still very underused in the industry, likely due to more burdensome test item requirements.
- Beagle dogs are often 5 to 10 kg at the start of a study, so here too is a greater chemistry, manufacturing, and controls strain. Regardless, they have been the default non-rodent species for many years.
- Although NHPs are often the obvious choice for biotherapeutics, homologous targets in rodents or transgenic mice can be considered as alternatives for safety testing.
Program delay or reprioritization
It is always an option to indefinitely delay a program — for example, by reprioritizing the development of another molecule for which other species are more appropriate. Of note, CROs cannot at this point guarantee either the future availability or the future prices of any NHPs. Therefore, re-evaluating program timelines could be a solution, depending on business considerations.
2022 Update
The shortage of NHPs has unfortunately not been entirely remedied since 2020. Although it is now possible to obtain the required animals, they often come with significant cost increases compared to pre-pandemic prices as well as consequential waiting times for emerging biotech. Of note, the US government is “investing heavily to breed more monkeys at the national facilities that house primates for biomedical research,” but it is not clear whether these efforts will have a strong impact on animal availability in the industry sector.
With deep nonclinical expertise, Premier Consulting provides solutions to help you design, execute, and manage your nonclinical program. If global shortages are impacting your development timeline or budget, the experts at Premier Partners and our network of CROs can help you to determine alternatives which could be viable options for your studies. Contact us today.