2019 505(b)(2) NDA Approvals in Review
In 2019, CDER approved 64 NDAs that used the 505(b)(2) pathway, representing important advances in patient care across a wide range of therapeutic areas.
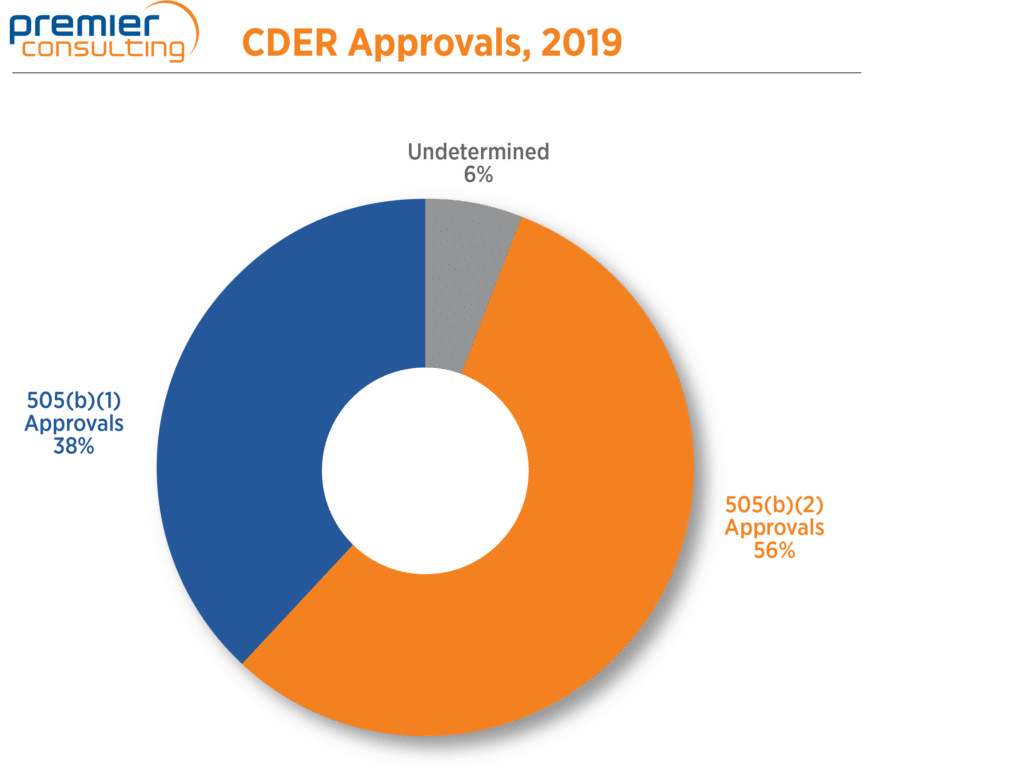
The number of 2019 NDA approvals that used the 505(b)(2) pathway fell from 75 in 2018 to 64 in 2019 (Figure 1), in line with a 13% drop in overall NDA approvals through FDA’s Center for Drug Evaluation and Research (CDER) 1. Despite the drop, 505(b)(2) NDA approvals continue to make up more than half (56%) of all NDA approvals through CDER 1,2 (Figure 2).
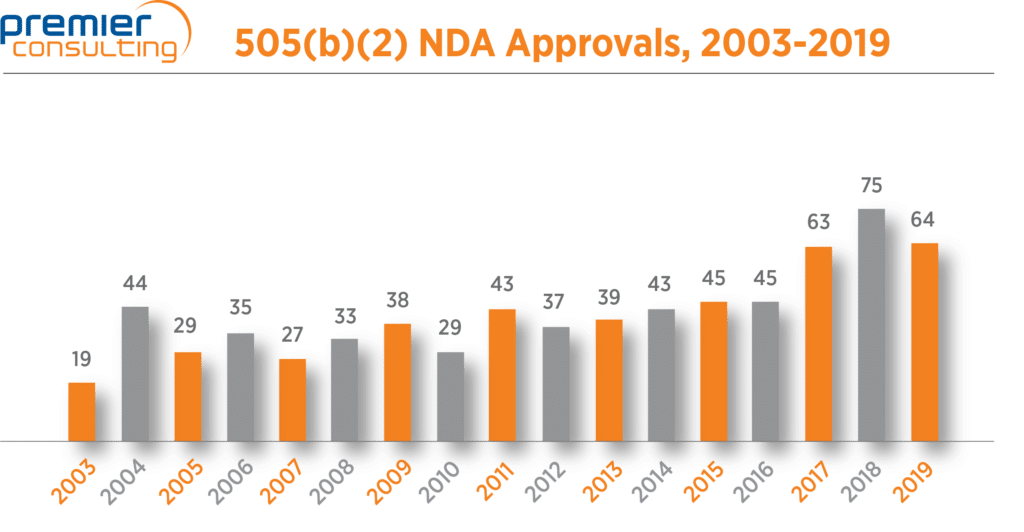
Figure 1
1This excludes BLA approvals and medical gas products approved through CDER.
2Number to date; however, review documents are not yet available to confirm the regulatory approval pathway of seven approved NDAs.
Figure 2
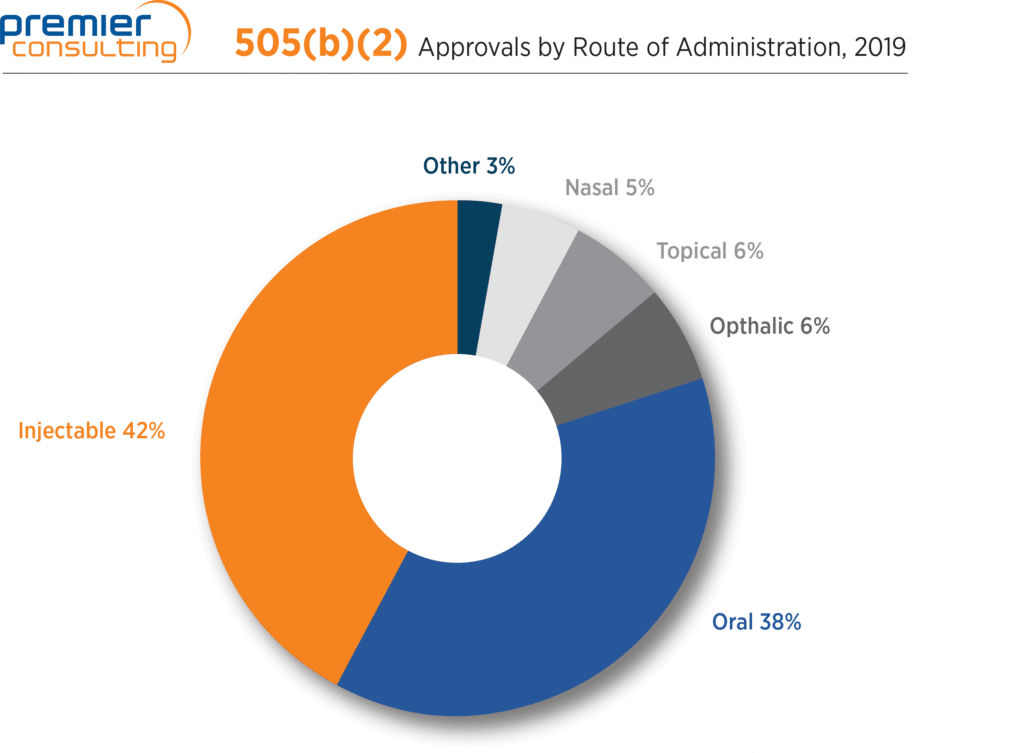
Injectable and oral routes of administration most common
Two routes of administration led the way for products approved through the 505(b)(2) pathway in 2019: injectable forms accounted for 42% of approvals, followed by oral forms at 38% (Figure 3).
Figure 3
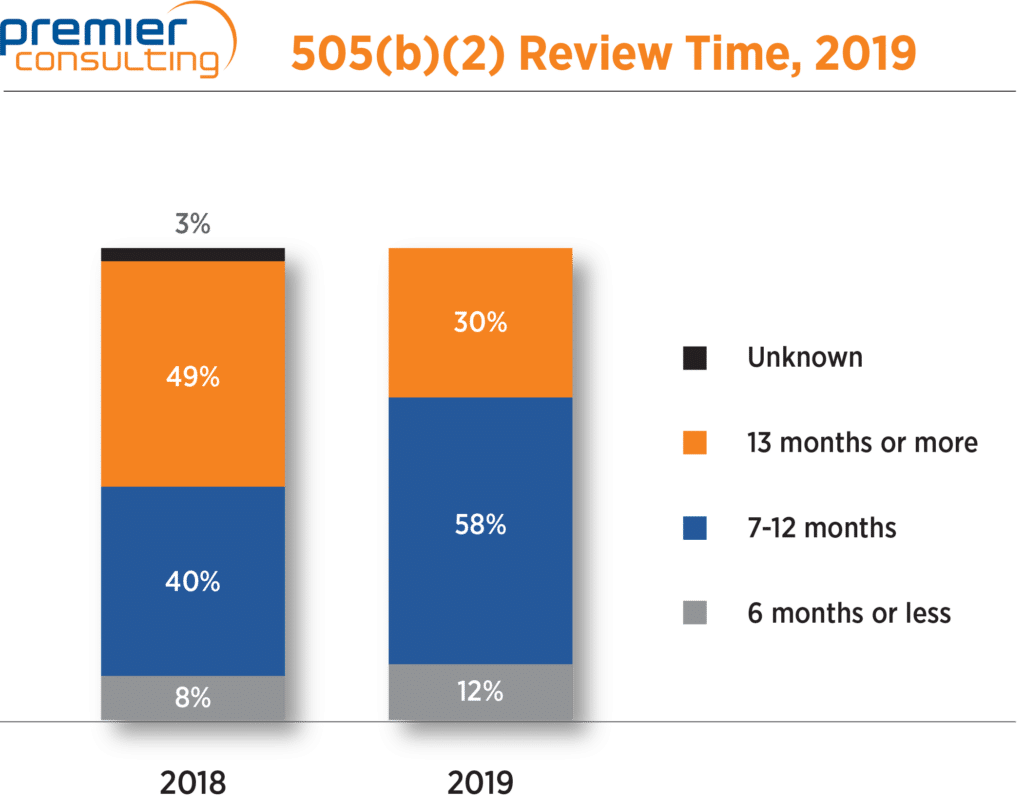
Mediam review time for 505(b)(2) NDAs drops
The median review time, which jumped from 10 to 13 months in 2018, was back down to 10 months in 2019. Overall, 71% of 505(b)(2) NDA approvals had review times 12 months or less, compared to 48% in 2018. Eight 505(b)(2) NDAs had review times of six months or less, five of which were priority review products (Figure 4).
| 2017 | 2018 | 2019 | |
|---|---|---|---|
| Median review time | 10 | 13 | 10 |
Figure 4
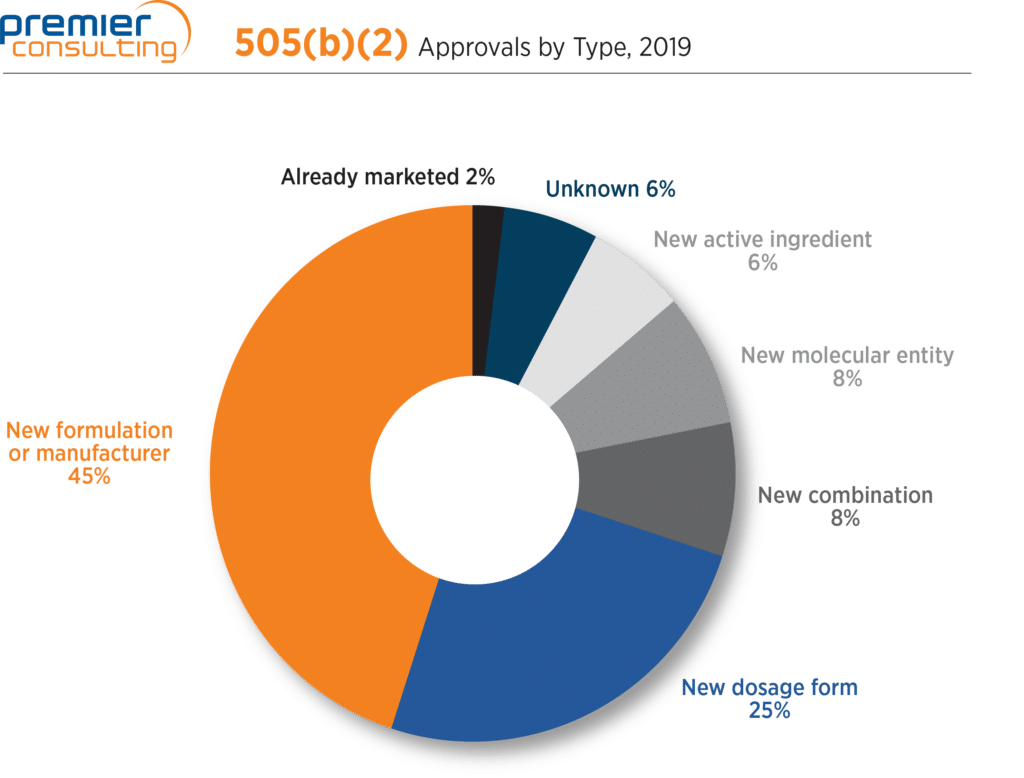
Top two submission classification types remain constant
Accounting for 70% of all 505(b)(2) approvals, (1) new formulation or manufacturer and (2) new dosage form remain the top two submission classification types, a trend which dates back 15 years (Figure 5).
Figure 5
New molecular entity category climbs to 8%
The number of 505(b)(2) approvals classified as new molecular entities (NMEs) grew from two (3%) in 2018 to five (8%) 2019. These approvals emphasize the potential for Sponsors to achieve NME classification and associated marketing exclusivity (more on that here) through the 505(b)(2) pathway. They are:
- Accrufer (ferric maltol; Shield Therapeutics)
Approved by the Office of Hematology and Oncology Products for the treatment of iron deficiency in adults. - Ga 68 DOTATOC (University of Iowa Hospitals and Clinics PET Imaging Center)
Approved by the Office of Drug Evaluation IV for use with positron emission tomography (PET) for localization of somatostatin receptor positive neuroendocrine tumors (NETs) in adult and pediatric patients. Ga 68 DOTATOC was granted Orphan Drug Designation. - Fluorodopa F-18 (Feinstein)
Approved by the Office of Drug Evaluation IV for use in positron emission tomography (PET) to visualize dopaminergic nerve terminals in the striatum for the evaluation of adult patients with suspected Parkinsonian syndromes (PS). Fluorodopa F 18 PET is an adjunct to other diagnostic evaluations. - Exem Foam (air polymer-type A; Giskit B.V.)
Approved by the Division of Medical Imaging Products intrauterine foam as an ultrasound contrast agent indicated for sonohysterosalpingography to assess fallopian tube patency in women with known or suspected infertility - TissueBlue (Brilliant Blue G; Dutch Ophthalmologic Research)
Approved by the Office of New Drugs for use as an aid in ophthalmic surgery by selectively staining the internal limiting membrane (ILM). TissueBlue was granted Priority Review and Orphan Drug Designation.
Five products add significant value for patients
From the products approved in 2019 through the 505(b)(2) pathway, we selected five products that present a high added value for patients:
- Jatenzo (testosterone softgel capsules; Clarus)
Testosterone is used as a replacement therapy in males with deficiency or absence of endogenous testosterone. Until the approval of Jatenzo, testosterone was available in the US only in topical or injectable forms. The capsule form has the potential to increase adherence and avoid transfer contamination over other available forms. - Talicia (delayed release capsules of amoxicillin, omeprazole magnesium, rifabutin; Redhill)
Talicia is a novel oral formulation that combines the antibiotics rifabutin and amoxicillin with the proton pump inhibitor omeprazole for the treatment of Helicobacter pylori infection in adults. Talicia offers patients a much-needed new treatment option for H. pylori with an excellent safety and efficacy profile that is not compromised by clarithromycin or metronidazole resistance, which are the antibiotics usually used to treat H. pylori. - Nayzilam (midazolam nasal spray; UCB)
Nayzilam is a rescue medication used for the short-term treatment of seizure clusters (also known as acute repetitive seizures). With Nayzilam, there is no need to wait to treat a seizure cluster. It can be given during or immediately after the first seizure if a cluster occurs, even if the person is unconscious. - Secuado (asenapine transdermal system; Hisamitsu)
Secuado, which is indicated for the treatment of schizophrenia in adults, may provide several advantages over sublingual asenapine, including reduced risk of oral hypoesthesia and dysgeusia, steadier absorption and plasma levels of asenapine, improved adherence to therapy, reduced potential for drug-drug interactions and improved tolerability (e.g. decreased gastrointestinal irritation). - Hemady (dexamethasone 20 mg tablets; Dexcel Pharma)
Dexamethasone is a widely used anti-inflammatory steroid, originally approved in 1958, and now available in many dosage forms used to treat different conditions such as allergic disorders, skin conditions, ulcerative colitis, arthritis, lupus, psoriasis, and breathing disorders. This new formulation has been approved to be used in combination with other medications to treat patients with multiple myeloma.
Want to know more about 2019 505(b)(2) approvals?
From which FDA division approved them, to who brought them to market, download our Sponsor Insights Infographic and take a deeper dive into the 64 505(b)(2) NDA approvals of 2019.
About the data
Data in this post comes from Premier Consulting’s proprietary 505(b)(2) database, and from FDA’s public databases including the Orange Book and drugs@FDA. Our 505(b)(2) database is maintained thanks to regulatory scientist Seth DePuy, PhD, in close collaboration with Angela Drew, PhD, Product Ideation Consultant. Ana Gavaldá, PhD, Business Development Consultant, selected the five products making an impact on patient outcomes and was a key contributor to this article.
Information is current as of February 10, 2020. Note that review documents are not yet available to confirm the regulatory approval pathway of seven approved NDAs.